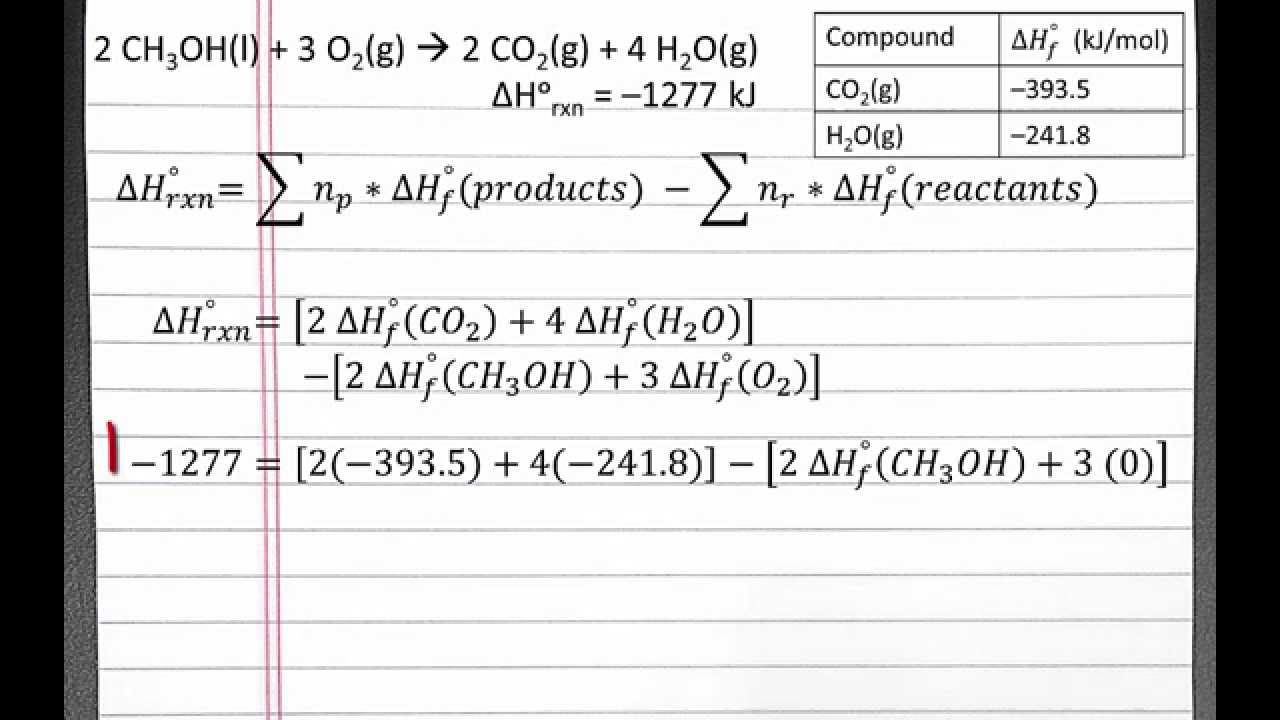
Standard Enthalpy Of Formation And Reaction Hess Law . standard enthalpies of formation. in this case, we are going to calculate the enthalpy change for the reaction between ethene and hydrogen chloride gases to make chloroethane gas from the standard. hess' law allows the enthalpy change (δh) for a reaction to be calculated even when it cannot be measured directly. the formation of co 2 (g) from its elements can be thought of as occurring in two steps, which sum to the overall reaction, as. a standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. The formation of co 2 (g) from its elements can be thought of as occurring in two steps, which sum to the overall reaction, as. the formation of co 2 (g) from its elements can be thought of as occurring in two steps, which sum to the overall reaction, as described by hess’s law. Standard enthalpies of formation help us predict reaction enthalpies for many.
from www.youtube.com
the formation of co 2 (g) from its elements can be thought of as occurring in two steps, which sum to the overall reaction, as. in this case, we are going to calculate the enthalpy change for the reaction between ethene and hydrogen chloride gases to make chloroethane gas from the standard. Standard enthalpies of formation help us predict reaction enthalpies for many. the formation of co 2 (g) from its elements can be thought of as occurring in two steps, which sum to the overall reaction, as described by hess’s law. The formation of co 2 (g) from its elements can be thought of as occurring in two steps, which sum to the overall reaction, as. a standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. standard enthalpies of formation. hess' law allows the enthalpy change (δh) for a reaction to be calculated even when it cannot be measured directly.
CHEMISTRY 101 Standard Enthalpy of reaction from Standard Enthalpies of Formation YouTube
Standard Enthalpy Of Formation And Reaction Hess Law The formation of co 2 (g) from its elements can be thought of as occurring in two steps, which sum to the overall reaction, as. hess' law allows the enthalpy change (δh) for a reaction to be calculated even when it cannot be measured directly. a standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. in this case, we are going to calculate the enthalpy change for the reaction between ethene and hydrogen chloride gases to make chloroethane gas from the standard. The formation of co 2 (g) from its elements can be thought of as occurring in two steps, which sum to the overall reaction, as. the formation of co 2 (g) from its elements can be thought of as occurring in two steps, which sum to the overall reaction, as described by hess’s law. the formation of co 2 (g) from its elements can be thought of as occurring in two steps, which sum to the overall reaction, as. standard enthalpies of formation. Standard enthalpies of formation help us predict reaction enthalpies for many.
From www.numerade.com
SOLVED 'experimentully determined heats (10) Apply Hess' Law t0 find the Heat of Formation for Standard Enthalpy Of Formation And Reaction Hess Law The formation of co 2 (g) from its elements can be thought of as occurring in two steps, which sum to the overall reaction, as. the formation of co 2 (g) from its elements can be thought of as occurring in two steps, which sum to the overall reaction, as. Standard enthalpies of formation help us predict reaction enthalpies. Standard Enthalpy Of Formation And Reaction Hess Law.
From www.thesciencehive.co.uk
Enthalpy Changes — the science hive Standard Enthalpy Of Formation And Reaction Hess Law a standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. the formation of co 2 (g) from its elements can be thought of as occurring in two steps, which sum to the overall reaction, as described by hess’s law. hess' law allows the enthalpy change (δh). Standard Enthalpy Of Formation And Reaction Hess Law.
From www.youtube.com
Hess's Law Chemistry Tutorial YouTube Standard Enthalpy Of Formation And Reaction Hess Law hess' law allows the enthalpy change (δh) for a reaction to be calculated even when it cannot be measured directly. a standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. the formation of co 2 (g) from its elements can be thought of as occurring in. Standard Enthalpy Of Formation And Reaction Hess Law.
From gbu-presnenskij.ru
Hess' Law OCR A Level Teaching Resources, 43 OFF Standard Enthalpy Of Formation And Reaction Hess Law Standard enthalpies of formation help us predict reaction enthalpies for many. standard enthalpies of formation. in this case, we are going to calculate the enthalpy change for the reaction between ethene and hydrogen chloride gases to make chloroethane gas from the standard. The formation of co 2 (g) from its elements can be thought of as occurring in. Standard Enthalpy Of Formation And Reaction Hess Law.
From studylib.net
5.2 b) HESS`S LAW AND STANDARD ENTHALPIES OF REACTION Standard Enthalpy Of Formation And Reaction Hess Law Standard enthalpies of formation help us predict reaction enthalpies for many. standard enthalpies of formation. a standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. The formation of co 2 (g) from its elements can be thought of as occurring in two steps, which sum to the. Standard Enthalpy Of Formation And Reaction Hess Law.
From slideplayer.com
Chapter 4 Thermochemistry. ppt download Standard Enthalpy Of Formation And Reaction Hess Law Standard enthalpies of formation help us predict reaction enthalpies for many. in this case, we are going to calculate the enthalpy change for the reaction between ethene and hydrogen chloride gases to make chloroethane gas from the standard. hess' law allows the enthalpy change (δh) for a reaction to be calculated even when it cannot be measured directly.. Standard Enthalpy Of Formation And Reaction Hess Law.
From www.youtube.com
Hess's Law Problems & Enthalpy Change Chemistry YouTube Standard Enthalpy Of Formation And Reaction Hess Law in this case, we are going to calculate the enthalpy change for the reaction between ethene and hydrogen chloride gases to make chloroethane gas from the standard. The formation of co 2 (g) from its elements can be thought of as occurring in two steps, which sum to the overall reaction, as. the formation of co 2 (g). Standard Enthalpy Of Formation And Reaction Hess Law.
From www.coursehero.com
[Solved] Calculate standard enthalpy change of reaction via Hess's Law. Use... Course Hero Standard Enthalpy Of Formation And Reaction Hess Law the formation of co 2 (g) from its elements can be thought of as occurring in two steps, which sum to the overall reaction, as described by hess’s law. the formation of co 2 (g) from its elements can be thought of as occurring in two steps, which sum to the overall reaction, as. a standard enthalpy. Standard Enthalpy Of Formation And Reaction Hess Law.
From www.slideserve.com
PPT Hess’s Law PowerPoint Presentation, free download ID6793985 Standard Enthalpy Of Formation And Reaction Hess Law The formation of co 2 (g) from its elements can be thought of as occurring in two steps, which sum to the overall reaction, as. the formation of co 2 (g) from its elements can be thought of as occurring in two steps, which sum to the overall reaction, as. in this case, we are going to calculate. Standard Enthalpy Of Formation And Reaction Hess Law.
From exoyrbcod.blob.core.windows.net
Heats Of Reaction And Hess's Law Lab at Sam Edwards blog Standard Enthalpy Of Formation And Reaction Hess Law the formation of co 2 (g) from its elements can be thought of as occurring in two steps, which sum to the overall reaction, as. The formation of co 2 (g) from its elements can be thought of as occurring in two steps, which sum to the overall reaction, as. Standard enthalpies of formation help us predict reaction enthalpies. Standard Enthalpy Of Formation And Reaction Hess Law.
From www.slideserve.com
PPT Thermochemisty (Enthalpy) and Hess’s Law PowerPoint Presentation ID3527437 Standard Enthalpy Of Formation And Reaction Hess Law the formation of co 2 (g) from its elements can be thought of as occurring in two steps, which sum to the overall reaction, as. in this case, we are going to calculate the enthalpy change for the reaction between ethene and hydrogen chloride gases to make chloroethane gas from the standard. the formation of co 2. Standard Enthalpy Of Formation And Reaction Hess Law.
From www.slideserve.com
PPT Enthalpies of Reactions and Hess’ Law PowerPoint Presentation, free download ID4494151 Standard Enthalpy Of Formation And Reaction Hess Law The formation of co 2 (g) from its elements can be thought of as occurring in two steps, which sum to the overall reaction, as. a standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. standard enthalpies of formation. hess' law allows the enthalpy change (δh). Standard Enthalpy Of Formation And Reaction Hess Law.
From studylib.net
Standard Enthalpies of Formation and Hess' Law Standard Enthalpy Of Formation And Reaction Hess Law Standard enthalpies of formation help us predict reaction enthalpies for many. the formation of co 2 (g) from its elements can be thought of as occurring in two steps, which sum to the overall reaction, as. The formation of co 2 (g) from its elements can be thought of as occurring in two steps, which sum to the overall. Standard Enthalpy Of Formation And Reaction Hess Law.
From study.com
Using Hess's Law to Calculate the Enthalpy of a Reaction Chemistry Standard Enthalpy Of Formation And Reaction Hess Law standard enthalpies of formation. the formation of co 2 (g) from its elements can be thought of as occurring in two steps, which sum to the overall reaction, as. Standard enthalpies of formation help us predict reaction enthalpies for many. The formation of co 2 (g) from its elements can be thought of as occurring in two steps,. Standard Enthalpy Of Formation And Reaction Hess Law.
From www.bartleby.com
Answered 7. Given the heats of the following… bartleby Standard Enthalpy Of Formation And Reaction Hess Law a standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. hess' law allows the enthalpy change (δh) for a reaction to be calculated even when it cannot be measured directly. in this case, we are going to calculate the enthalpy change for the reaction between ethene. Standard Enthalpy Of Formation And Reaction Hess Law.
From university.pressbooks.pub
10.4 Hess’s Law Chemistry Fundamentals Standard Enthalpy Of Formation And Reaction Hess Law a standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. Standard enthalpies of formation help us predict reaction enthalpies for many. the formation of co 2 (g) from its elements can be thought of as occurring in two steps, which sum to the overall reaction, as described. Standard Enthalpy Of Formation And Reaction Hess Law.
From www.numerade.com
SOLVED Use Hess's Law and the thermochemical equations labelled 1 and 2 below, and their Standard Enthalpy Of Formation And Reaction Hess Law in this case, we are going to calculate the enthalpy change for the reaction between ethene and hydrogen chloride gases to make chloroethane gas from the standard. the formation of co 2 (g) from its elements can be thought of as occurring in two steps, which sum to the overall reaction, as. hess' law allows the enthalpy. Standard Enthalpy Of Formation And Reaction Hess Law.
From surfguppy.com
Calculate the enthalpy change of methane formation using Hess Law Surfguppy Chemistry made Standard Enthalpy Of Formation And Reaction Hess Law Standard enthalpies of formation help us predict reaction enthalpies for many. The formation of co 2 (g) from its elements can be thought of as occurring in two steps, which sum to the overall reaction, as. in this case, we are going to calculate the enthalpy change for the reaction between ethene and hydrogen chloride gases to make chloroethane. Standard Enthalpy Of Formation And Reaction Hess Law.